
DISCLAIMER
Please note: The information contained in this section is restricted to medical and healthcare practitioners for general education and information purpose only

Mefenamic Acid, an N (2,3-xylyl) anthranilic acid, was initially released in 1962 for pharmaceutical purposes and was marketed in several countries.
t is available in many European and South-East Asian countries, including India.
The drug possesses anti-pyretic, analgesic, and anti-inflammatory properties.
One of the most highly cited drugs in the existing scientific and medical literature, fenamates are one of the most highly studied drugs.
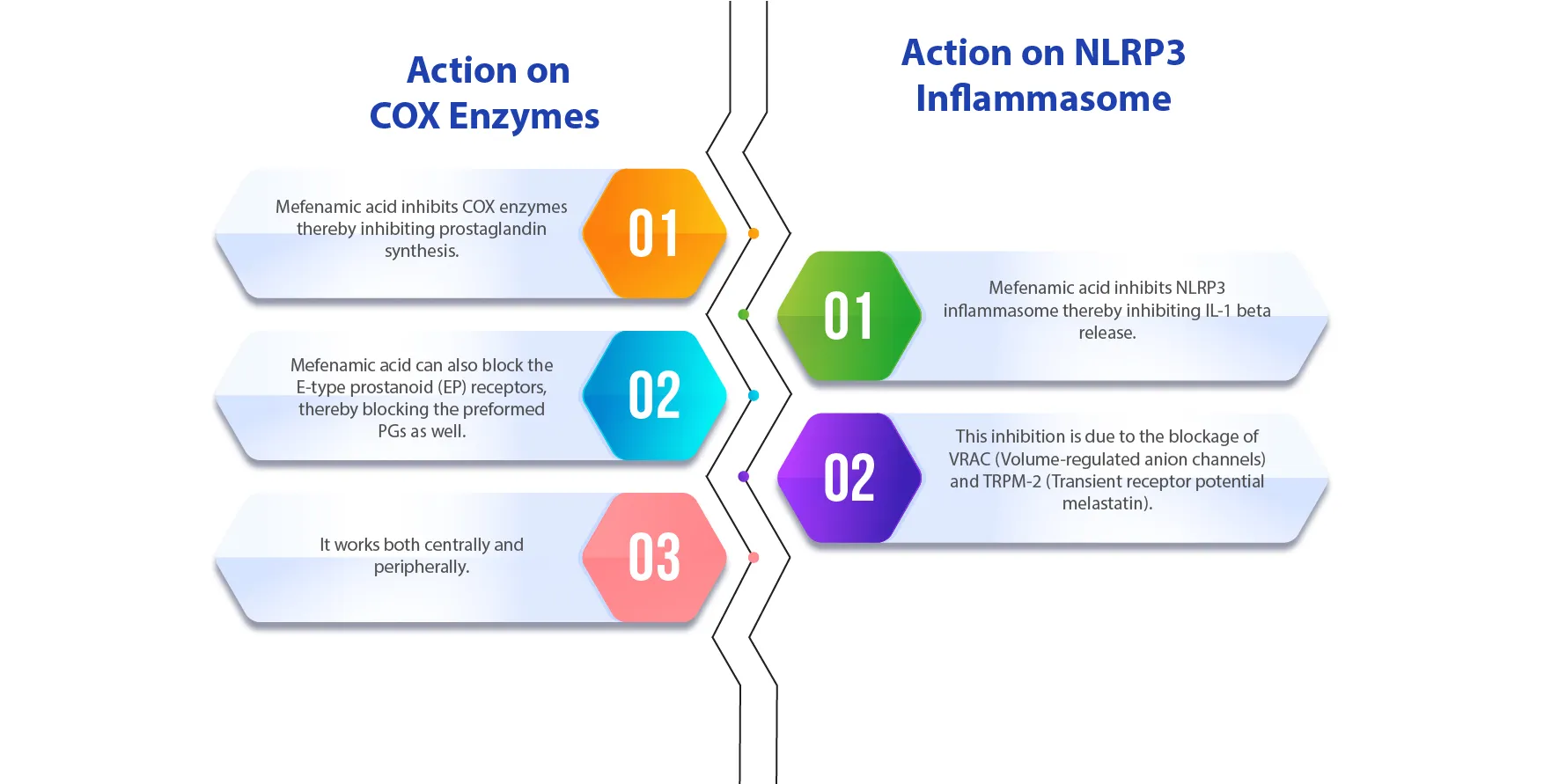
In addition to PG synthesis inhibition, what is unique about Mefenamic Acid is its ability to block the E-type prostanoid (EP) receptors, thus blocking the pre-formed PGs as well.
It is a competitive, time-dependent, and reversible inhibitor for COX-1 and COX-2.
It is the only anti-pyretic with dual action on COX inhibition (central and peripheral mechanisms) and NLRP3 inflammasome inhibition.
This study showed that the NLRP3 inflammasome inhibitory action of Mefenamic Acid may attenuate the levels of Pyrogenic Cytokines (IL-1β).1
A case series including 71 patients from 3 months to 15 years of age showed that Mefenamic Acid given in a 4 mg/Kg dose resulted in optimal anti-pyretic effect. 5
The anti-pyretic effect was 2.5 times that of Paracetamol. 5
References
References

